To receive the most up to date information, please sign up for my newsletter and follow me on Facebook, Instagram, and Twitter.
The Trump Administration announced that it will be pushing back the tax filing deadline to July 15, 2020.
The Trump Administration and Congress have taken important steps to ensure the risk of infection for Americans remains low. In addition to monitoring travelers coming into the United States, the Administration has declared a public health emergency and has utilized reserved funding to help support response efforts to the virus, including increased education to identify cases. The CDC has been working with state and local officials to make sure they are prepared to deal with any new development that may arise. The Administration is also working to expedite the development of a vaccine, therapeutics and monoclonal antibodies that can be used both to protect from infection, as well as treat people already infected and is consulting experts and expanding research to better understand the transmission of Coronavirus.
Federal Actions:
- The Administration declared a public health emergency and utilized reserved funding to help support response efforts to the virus, including increased education to identify cases
- The Administration imposed travel restrictions on most non-citizens coming from China and Iran to America while also instituting a mandatory quarantine of Americans returning from China
- Airport screenings have been expanded to identify anyone showing symptoms and monitoring travelers coming into the United States from at-risk nations
- The Centers for Disease Control and Prevention (CDC) has mobilized state health departments to receive returned travelers and are working with state and local officials to make sure they are prepared to deal with any new development that may arise
- 62 Public Health Emergency Preparedness programs are part of the multi-agency infrastructure working on quarantine, isolation, case finding, protecting health care workers, and assuring medical supply chains.
- The Federal Emergency Management Agency (FEMA) is readying over fifty teams to respond and support states and territories that may need assistance.
- The Department of Housing and Urban Development (HUD) is communicating with public housing authorities and homeless service providers.
- The Administration is working to expedite the development of a vaccine, therapeutics and monoclonal antibodies that can be used both to protect from infection, as well as treat people already infected and consulting experts and expanding research to better understand the transmission of coronavirus
- All levels of government are consulting experts and expanding research to better understand the transmission of Coronavirus
On March 4th, the House approved over $7 billion in emergency funding for the U.S. response to the Coronavirus. This legislation does not contain any policy riders or political poison pills, and is the product of a bipartisan compromise to address this emergency. This bill provides important funding that allows the Administration to address the virus at home and abroad, including expediting vaccine development, purchasing essential equipment and supplies, and assisting state and local health departments. Over half of the funding will go to make diagnostic tests more widely available, to support treatments to ease symptoms of those infected, and vaccine development.
On March 5th, the Senate cleared an $8.3 billion emergency funding bill in a 96-1 vote to combat the Coronavirus, sending the measure on for President Donald Trump's signature. The bill, H.R. 6074 (116), will kick almost $6.5 billion to the Department of Health and Human Services, nearly $1.3 billion to the State Department and $20 million to the Small Business Administration, as the federal government rallies to aid state and local response to the virus that has sickened more than 160 people in more than a dozen states.
I also participated in a conference call with the Virginia Department of Health who outlined the most up to date action plan regarding COVID-19. While the risk is still low, state agencies are focused on preparation efforts, and are encouraging Virginians to practice good hygiene measures. Potential cases of COVID-19 will be tested at Virginia’s Division of Consolidated Laboratory Services, rather than being tested at the Centers for Disease Control (CDC) in Atlanta, Georgia. Virginia-based testing is expected to generate results within a few hours, allowing for faster responses.
On March 6th, President Trump signed an $8.3 billion spending bill aimed at fighting the coronavirus outbreak, the culmination of a bipartisan effort by Congress and the White House to provide funds to federal agencies, as well as state and local governments, to battle the disease. “We’re doing well, but it’s an unforeseen problem,” the president said as he signed the legislation in the White House's Diplomatic Reception Room. Vice President Mike Pence announced Thursday that Trump would sign the $8.3 billion spending bill to fund the fight against the coronavirus on Friday. The legislation passed overwhelmingly in both the House of Representatives and the Senate in a rare show of bipartisanship in a very polarized Congress. The vice president said the efforts by health officials and lawmakers represented the “very best of D.C. coming together, putting the health and wellbeing of the American people first and making nearly $8 billion available not only to federal agencies but to state and local efforts as we confront coronavirus.”
During the week of March 16th, the Senate passed and the President signed into law legislation that originated in the House to address the Coronavirus outbreak across the United States. Click here for the update I sent out about that bill with key information for small businesses and employees.
Late Thursday, March 19th, Senate Majority Mitch McConnell formally introduced phase three of coronavirus response legislation. The Leader repeated that the Senate will not leave Washington until they pass a bill.
This bill is an important first step in the process to reaching a final relief package. It is focused on four main priorities:
1. Direct financial help for the American people;
2. Rapid relief for small businesses and their employees;
3. Significant steps to stabilize our economy and protect jobs;
4. And, of course, more support for the brave healthcare professionals and the patients who are fighting the coronavirus on the front lines.
The American worker, family, and business owner are counting on their government to act with speed and precision in this time of extraordinary need. Members of the House have been in regular contact with our Senate colleagues and I applaud this collaborative approach, which will continue as this package evolves and is refined. Just this week I was on a phone conference with Governor Northam, both Senators Warner and Kaine, and the entire Virginia House delegation to share information and discuss the best path forward for Virginians. Together is how we must govern in times of consequence to ensure we act quickly and effectively.
During Thursday's White House Coronavirus Task Force briefing, President Trump announced that his Administration was taking steps to cut red tape to empower coronavirus response efforts. Specifically, he and FDA Commissioner Stephen Hahn said that new action would empower certain qualified laboratories to use validated coronavirus tests while their Emergency Use Authorization requests were under review, and would empower states to authorize tests developed and used within their borders.
Also, the State Department released a statement Thursday advising U.S. citizens to avoid all international travel due to the global impact of COVID-19.
Be sure to check out these graphics below for guidance on social distancing and travel, as well as a breakdown of resources available to folks impacted economically and a look at the response of various federal agencies and Congress to address this constantly evolving situation.
White House coronavirus guidelines for Americans:
Actions by the Department of Defense to support the Administration’s pandemic response efforts:
Work being done to ensure the safety and soundness of economic and financial institutions:
Actions taken by Congress and the Trump Administration to address the Coronavirus that fall under the purview of the Energy and Commerce Committee:
Information and resources to ensure the safety and security of those who are abroad:
These are just a few of the many resources available to folks during these difficult times. Click here for more information on the Coronavirus from the CDC and click here for the Virginia-specific health response to COVID-19 from the Virginia Department of Health.
Government Preparedness
The Federal government has been preparing for public health challenges such as this. In fact, a recent Johns Hopkins study, the 2019 Global Health Security Index, ranks the United States number one in a “comprehensive assessment and benchmarking of health security and related capabilities across the 195 countries.” This is largely due to legislation passed under a Republican-led House in the previous years.
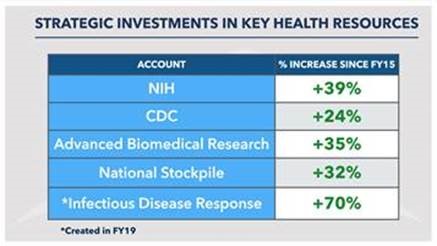
Since 2015, Congressional Republicans have increased funding to public health agencies - including a 70% increase to Federal funding to infectious disease response. Specific increases included below:
(NOTE: NIH, CDC, and ASPR totals about $50 billion a year. We are ready)
In addition to funding, last summer, the President signed the Pandemic and All-Hazards Preparedness Act.